Temperature and humidity monitoring scheme for medical storeroom GSP
Product description:
I. importance and development trend of temperature and humidity monitoring
as a special commodity, medicine is directly related to people's life and health problems. How to ensure the quality and safety of drugs in the aspects of drug production, transportation and storage is an important issue that the government departments at charge of drug administration and pharmaceutical enterprises at all levels are concerned about. As an important part of drug circulation, drug storage plays an important role in the process of drug circulation, and it is also the focus of drug administration at present.
as we all know, the scientific and reasonable temperature and humidity environment is the first condition to ensure the safety of drug storage. The GSP has issued new regulations, on how to regulate the drug warehouse temperature and humidity of the environment, made clear: "enterprises should set the temperature and humidity monitoring system, the automatic monitoring and data acquisition of temperature and humidity environment, the implementation of 24 hours of continuous monitoring, automatic and real-time recording of temperature and humidity. Each warehouse (or warehouse) should be equipped with a number of temperature and humidity monitoring equipment, used for the automatic monitoring and data collection of the temperature and humidity in the warehouse." In the past few years, the traditional way of monitoring temperature and humidity are temperature and humidity monitoring system of intelligent and automation, paperless and network replaced in can be expected in the next few years, all of China's pharmaceutical production and operation of enterprises will change the monitoring of temperature and humidity, temperature and humidity monitoring system intelligent assembly, in order to meet the national drug regulatory requirements and policies.
from the drug regulatory policies and the national drug safety management and pharmaceutical production enterprises GSP certification smoothly through the consideration, the call of the times appear more intelligent temperature and humidity monitoring system. Temperature and humidity monitoring has played an important role in ensuring the quality and safety of drugs in production, transportation and storage.
Two, temperature and humidity monitoring requirements and objectives
(1) GSP and GMP certified temperature and humidity monitoring system
development and application of China has over the years the company has been committed to the field of monitoring temperature and humidity, temperature and humidity monitoring in the pharmaceutical industry has accumulated rich experience, the large temperature and humidity monitoring system used in the national pharmaceutical enterprises. The relevant provisions of our combination of medical GSP, GMP certification, targeted to launch the industry temperature and humidity monitoring system of medicine, in order to meet the relevant provisions of the pharmaceutical production and business enterprises to monitor temperature and humidity reached GSP, GMP certification, in order to assist enterprises smoothly through the relevant certification.
(2) temperature and humidity monitoring requirements
using modern sensor technology, automatic control technology, digital communication technology, computer technology and other multi-disciplinary comprehensive application, environmental temperature and humidity monitoring will require several areas within the automatic measurement, and the data of the 24 hour uninterrupted communication wired or wireless transmission to the monitoring the computer realization for each data partition management, such as software, check the real-time data and alarm alarm, data record, data storage and export and permanent preservation, and combining with the temperature and humidity field monitoring and automatic control of the field temperature and humidity control equipment, thus realizing the intelligent automation system and the network temperature and humidity monitoring system, to provide comprehensive and practical for the pharmaceutical industry, temperature and humidity monitoring solutions.
(3) the goal of system construction
The construction of a stable, reliable and practical, temperature and humidity monitoring system has certain forward-looking and upgrade space: through the system, so that the temperature and humidity of scientific and standardized management, energy efficient, intelligent and practical, meet in a certain period of time, temperature and humidity monitoring of medical GSP in GMP certification requirement, in order to better for medicine the majority of the production and business enterprises to provide more comprehensive and standardized in terms of temperature and humidity monitoring service.
(4) layout requirements;
drug temperature library temperature is 0-30 degrees; cool storehouse temperature is not higher than 20 degrees; cold storage temperature is 2-10 degrees; warehouse relative humidity should be maintained between 45-75%.
Cool storage room temperature Library: flat warehouse every 200 square meters should not be less than 1 monitoring points, on the basis of 200 square meters, each increase of 200 square meters to increase a measuring point (increase the area of less than 200 square meters, increase by 200 square meters count).
Every 200 square meters of independent drug storehouse (or warehouse), storage facilities should be equipped with temperature and humidity monitoring equipment, used for environmental temperature and humidity automatic monitoring and data acquisition.
There are partitions in the library, and the length and height of the partition are greater than the length of the warehouse and the high 1/2. The two sides of the partition are regarded as independent warehouses
Cold storage: single base 20 square meters shall be not less than 2 monitoring points, 20-50 square meters shall be not less than 3 monitoring points, 50-150 square meters shall be not less than 4 monitoring points; point layout according to the cold temperature and hot point verification.
1 monitoring points of refrigerator and freezer. The enterprise take freezer split recorder fixed in the freezer, temperature probe, alarm records located outside the box body, connected with the GSP temperature and humidity control system of the enterprise, the direct transmission to the platform.
Three dimensional and elevated warehouse: should be distributed evenly in the upper, middle and lower levels of the 3 layers of the warehouse(10%-90%RH)
6) display mode: LCD display, with backlight, accuracy can be calibrated
7) control mode: 4 way control output, alarm value and alarm return difference can be set
8) communication mode: Standard MODBUS-RTU protocol, easy to communicate with all kinds of equipment networking, versatility
9) record function: with record function, USB port
3, system performance advantages
1) the system performance is stable, the design structure is scientific and standard, which meets the requirements of temperature and humidity monitoring in GSP and GMP certification.
2) system installation is simple, all functions of the menu, simple operation, man-machine interface design, the system functions at a glance, intuitive and clear.
3) system functionality, interface layout, comprehensive content, data / curve display, data record, query, flash alarm, automatic control, alarm message, dial alarm, electronic map and abundant customized functions can meet various needs of monitoring function.
4) system upgrade, system expansion, maintenance convenience, modular management, easy to upgrade software, system expansion and routine maintenance.
5) through the open database interconnection interface (ODBC) provided by the system, it can easily realize data interaction with other systems, and realize monitoring information sharing.
6) system operation experience for many years, improving the system structure and system function, flexible networking and communication, it can easily be different the most suitable way to monitor transmission of temperature and humidity monitoring platform of the centralized management center on the temperature and humidity monitoring site.
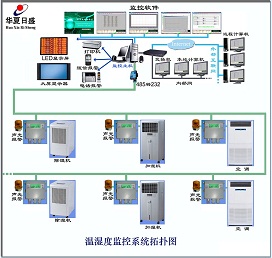
Four 、 temperature and humidity monitoring topology
